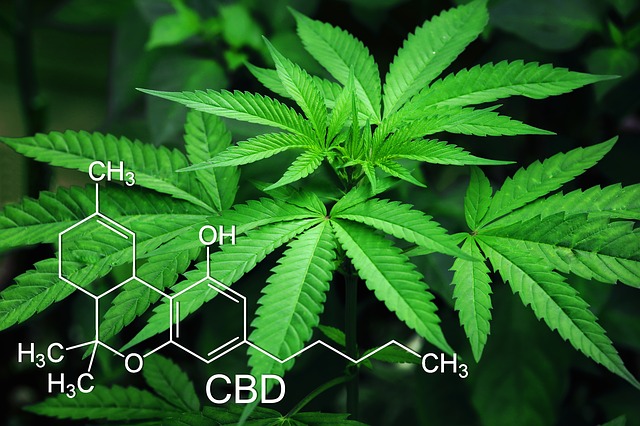
What is Cannabidiol?
Introduction:
Most of the people don’t know what Cannabidiol is? Cannabidiol or CBD is a Phytocannabinoid which was discovered in 1940. This natural chemical is one of 113 cannabinoids in cannabis plants. Furthermore, it accounts for up to forty (40) pc of the extracts of the plant. As of 2019, clinical research on CBD enclosed studies of anxiety, cognition, movement disorders, and pain. However, there’s still inadequate proof that it’s useful.
You can take Cannabidiol or CBD into the body in multiple ways. One way is by inhalation of cannabis smoke or vapor as an aerosol spray into the cheek and by mouth. It’s going to equip as CBD oil containing CBD solely because of the active ingredient. These ingredients (excluding tetrahydrocannabinol [T.H.C.] or terpenes), CBD-dominant hemp extract oil, capsules, dried cannabis, or liquid prescription solution. CBD doesn’t have the same psych activity as a psychoactive substance. It might modify psychoactive substances on the body if each is a gift. The mechanism and process of action for the biological effects of CBD did not determine as of 2018.
In the U.S.A., the cannabidiol drug Epidiolex was approved in 2018 to treat two epilepsy disorders. Since cannabis is a Schedule, I regularized material in the U.S.A., other CBD declaration, and formulation stay black-market. Below the federal law to instruct or command for medical purposes part in dietary supplements.
How is CBD Used?
CBD is obtained from Maryjane plants as either an oil or powder. You can blend it into creams or gels. You can place it into containers, taken orally, or scoured on your skin. How CBD ought to be utilized generally relies upon what will utilize. Converse with your P.C.P. before using CBD oil. It hasn’t been endorsed by the U.S. Food and Drug Administration (F.D.A.) for any clinical uses, and it can have reactions.
Chemistry of Cannabidiol:
At temperature, Cannabidiol may be a colorless crystalline solid. In powerfully primary media, it is also the presence of air. It’s altered to a quinone. Under acidic conditions, it cyclizes to the consciousness-altering drug. Which additionally happens during pyrolysis (smoking). Many analysis teams have accomplished the synthesis of Cannabidiol (CBD).
Biosynthesis of CBD or Cannabidiol:
Cannabis produces CBD-carboxylic acid through a similar metabolic pathway as a consciousness-altering drug, till ensuing to the last step, where CBDA synthase performs chemical change instead of THCA synthase.
A little About History of Cannabidiol:
Efforts to isolate the active component in cannabis were created within the nineteenth century. In 1940 Cannabidiol or CBD was studied from Minnesota wild hemp plant and resin od Egyptian Cannabis indica. The statement of CBD was planned from a technique for uninflected it from wild hemp. The structure and stereochemistry of it were determined, or you can say you set on in 1963.
Breeding of plant:
Selective breeding of cannabis plants has been distended and distributed as industrial and therapeutic markets develop. Some growers of this plant within the North American nation (the U.S.A. and others) succeeded in decreasing the CBD-to-THC proportion to facilitate customers and clients who most well-liked varietals lot psychotropic and psychoactive because of the upper consciousness-altering drug and lower CBD content. In the North American nation, hemp is classed by the federal as cannabis containing no over zero, 3% consciousness-altering drug by dry weight. This classification of CBD and T.H.C. in products was established and settled within the 2018 bill. It was also purified to make it part of hemp-sourced extracts, cannabinoids, and derivatives within the definition and explanation of hemp.
Medical Uses
Research:
As of 2019, there was solely restricted high-quality proof for Cannabidiol having a neurological effect in individuals, mainly thanks to the weak style and tiny variety of subjects in randomized controlled trials. CBD Oil For Anxeity
Epilepsy:
In 2018, Cannabidiol or CBD was FDA-approved (trade name Epidiolex) to treat two sorts of treatment-resistant epilepsy: Dravet syndrome and Lennox-Gastaut syndrome in kids with refractory epilepsy. The suggested daily dose of Epidiolex is 10 mg per kilogram weight per day in epileptic kids 2–5 years recent. While Epidiolex treatment is mostly right, it’s related to minor adverse effects, such as gastrointestinal upset, shriveled appetency, drowsiness and lethargy, and low sleep quality. Still.
Other uses:
Research that is base on the alternative uses for Cannabidiol or CBD includes several neurological disorders. However, the findings haven’t been finalized and confirmed to ascertain such uses in clinical practice. In October 2019, the Associate in Nursing Drug Administration, F.D.A., agency, federal agency, government agency, bureau, office authority issued a consultative warning. The consequences of CBD throughout physiological condition or breastfeeding are unknown, indicating that the security, doses, interactions with alternative medicine or foods, and side effects of CBD are not clinically defined and should create a risk to the mother and baby.
Non-intoxicating effects:
Cannabidiol doesn’t seem to own any intoxicating effects (i.e., “getting high”) such as those caused by ∆9-THC (Tetrahydro cannabidiol) in marijuana, however, is below first and foremost analysis for the possible anti-anxiety and anti-psychotic impacts of it. As the legal outlook and comprehension regarding the variations and differentiation in medical cannabinoids unfold, experts and professionals are working to distinguish marijuana. That is specific for medical purposes (with varying levels of psychotropic impacts and deficits in executive purpose and function) from “medical CBD therapies,” which might usually gift as having a reduced and low grade or non-psychoactive side-effect side of this product.
Different strains and spots of “medical marijuana” (marijuana that is specific for medical purposes) to have a significant). They are known to have other non-psychotropic cannabinoids. Any mind-bending marijuana, despite its CBD content, comes from the flower (or bud) of the genus Cannabis. As outlined by U.S.A. federal law, non-psychoactive hemp (also commonly-termed “industrial hemp”), despite its CBD content, is any a part of the cannabis plant not growing or not, containing a ∆9-tetrahydrocannabinol concentration of no quite zero.3% on a dry-weight basis. Specific standards are needed for legal developing, cultivating, and manufacturing the hemp plant. The Industrial Hemp of Colorado Industrial Hemp Program registers growers and farmers of business hemp and samples crops to testify that the dry-weight T.H.C. concentration doesn’t cross the limit of zero.3%.
Side effects:
Research indicates that Cannabidiol could reduce adverse effects of T.H.C., notably those inflicting intoxication and sedation, however solely at high doses. Safety studies of Cannabidiol showed it’s well-tolerated, yet it could cause fatigue, diarrhea, or changes in appetency as common adverse effects. Epidiolex documentation lists drowsiness, insomnia and poor-quality sleep, shriveled appetency, diarrhea, and fatigue.
Potential interactions:
Laboratory proof indicated that Cannabidiol could scale back T.H.C. clearance, increasing plasma concentrations, which can raise T.H.C. accessibility to receptors and enhance its impact in a dose-dependent manner. In vitro, Cannabidiol strangled receptors touching the activity of voltage-dependent sodium and potassium channels, affecting neural activity. Still. A little and small medical trial reported that the CBD part throttled the CYP2C-catalyzed hydroxylation of T.H.C. to 11-OH-THC. Little is thought concerning potential drug interactions; however, CBD mediates a decrease in clobazam metabolism.
Pharmacodynamics:
Cannabidiol has low affinity or harmony for the cannabinoid CB1 and CB2 receptors, although it will act as an antagonist of CB1/CB2 agonists despite this low affinity. Cannabidiol could also be an associate antagonist of GPR55, a G protein-coupled receptor, and a reputed cannabinoid receptor expressed in the caudate nucleus and putamen in the brain. It conjointly might act as an inverse agonist of GPR3, GPR6, and GPR12. CBD is shown (has been established) to act as a serotonin 5-HT1A receptor partial protagonist. It is an allosteric modulator and intonation of the μ- and δ-opioid receptors as well. The pharmacologic effects of CBD might involve PPARγ agonism and intracellular metal release. However, CBD mediates a decrease in clobazam metabolism.
Pharmacokinetics:
The oral bioavailability of Cannabidiol is roughly 6% in humans. Simultaneously, its bioavailability via inhalation is eleven to 45% (mean 31%). The elimination half-life (in which material is half left) of CBD is 18–32 hours. Cannabidiol is processed in the liver just as in the digestive organs by the cytochrome P450 compounds. CYP2B6 or CYP2C19 or CYP2D6 or CYP2J2, and CYP3A4, and by the isoenzymes UGT1A7, UGT1A9, and UGT2B7. CBD might have a large margin in dosing.
Pharmaceutical preparations:
Nabiximols (brand name Sativex), a proprietary drug containing Cannabidiol and mind-altering drug in equal proportions, was approved by Health North American nation in 2005 to treat central neuropathic pain in multiple sclerosis, and in 2007 for problem-related to cancer. In New Island, Sativex is “approved for use as AN add-on treatment for symptom improvement in folks with moderate to severe jerkiness because of disseminated sclerosis who haven’t responded adequately to different anti-spasticity medication.”
Epidiolex is an AN orally administered cannabidiol solution. It had been approved in 2018 by the U.S. Food and Drug Administration (F.D.A.) for the treatment of 2 rare styles of childhood brain disease, Lennox-Gastaut syndrome, and Dravet syndrome.
Side Effects as Major
CBD oil usually doesn’t have any significant risks for users. However, the side effects of cannabidiol oil are possible. These include: Still.
depression
dizziness
hallucinations
low blood pressure
withdrawal symptoms, such as irritability and insomnia
More human studies are needed to fully get the range and level of risks and side effects that CBD oil may cause. Reviews of CBD oil aren’t familiar. It is partially because Schedule 1 substances like cannabis are highly regulated, causing some obstacles for researchers. With the legalization of marijuana products, more research is possible, and more answers will come.
CBD (Cannabidiol) in society
Beverages and Food items:
Food and food products containing Cannabidiol were wide marketed inside the U.S.A. as early as 2017. Hemp seed ingredients that don’t naturally contain mind-altering drug or CBD (but might be contaminated and adulterer with trace amounts on the skin throughout harvesting) were declared. Known by the North American country Food and Drug Administration (F.D.A.) as usually and commonly recognized as safe (GRAS) in Dec 2018. CBD itself has not been declared or proclaimed GRAS and below the United States of America. Federal law is against the law to sell as it as a food, dietary supplement, or animal feed, and so on. State laws varied significantly as non-medical cannabis and derived products are legalized in some jurisdictions within the 2010 decade.
Similar gradeergy liquids and protein bars that could contain alimentation or flavoring additives, food, and nutrient things are infused with CBD as an alternate means that of ingesting the substance. In the U.S.A., various products are marketed as containing CBD; however, they contain very little or none. Some firms promoting CBD-infused food products with claims that are just like the consequences of prescription drugs have received warning letters from the Food and Drug Administration for creating unsubstantiated health claims. In February 2019, the New royal line City Department of Health declared plans to fine restaurants that sell food or drinks containing CBD, starting in the Gregorian calendar month 2019.
Cannabidiol in Sports:
Cannabidiol has been utilized by skilled and amateur athletes across disciplines and countries, with the World Anti-Doping Agency removing CBD from its banned substances list. The Anti-Doping Agency of United States and don’t have anti-CBD policies, with the latter (Anti-Doping Agency of United Kingdom) states that, “CBD or Cannabidiol is not currently in the list of or Prohibited List the World Anti-Doping Agency. As a result, it’s permissible to use in sport. All alternative cannabinoids (CBD) (including, however, not restricted to cannabis, hashish, marijuana, and T.H.C.) are strictly prohibited in the competition. The rules intend to ban cannabinoids that activate an equivalent receptor within the brain as activated by the mind-altering drug.”
In 2019, the leading cannabis product manufacturer, Canopy Growth, nonheritable majority possession of Bio Steel Sports Nutrition, develops a CBD product below endorsement by various skilled athletes. The National Hockey League Alumni Association started a project with cover Growth to see if CBD or alternative cannabis products would improve medical symptoms and quality of life in head-injured players. Usually, for treating pain, Numerous skilled athletes use CBD or, in other words, Cannabidiol.
Legal Status of Cannabidiol
Australia:
Prescription medication (Schedule 4) for therapeutic use containing a pair of % (2.0%) or less of alternative cannabinoids unremarkably found in cannabis (such as ∆9-THC). A schedule 4 drug beneath the SUSMP is a Prescription solely medication or Prescription Animal Remedy – Substances, the employment or provision of that should be by or on the order of persons allowable by State or Territory legislation to visit and will be obtainable from a chemist on prescription.
Following a modification in legislation in 2017, CBD was modified from a schedule 9 drug to a schedule 4 drug, which means that it’s de jure obtainable in Australia. However, most merchandise containing CBD is still to be approved by the Australian Register of Therapeutic inventory (ARTG). As of one Apr 2020, there are solely half-dozen medicines presently active on the ARTG that contain Cannabidiol as a lively ingredient. These are all export only medicines.
Bulgaria:
In 2020, the Republic of Bulgaria became the primary E.U. country to permit retail sales of food merchandise and supplements containing CBD. Still. Despite the continued discussion at intervals, the E.U. concerning the categorization of CBD (Cannabidiol) as a Novel food (food that does not have any significant history of food consumption).
Canada:
As of August 2019, CBD merchandise in Canada might solely be sold by approved retailers or federally commissioned medical firms, limiting their access to the general public. The Canadian government states that Cannabidiol products “are subject to all or any principles and requirements. That applies to cannabis under the Cannabis Act and its regulations.” It requires “a processing license to manufacture products containing CBD purchasable, no matter what the availability of the CBD is. Which CBD and merchandise containing CBD, like cannabis oil, may only be sold” by a licensed retailer or licensed seller of medical CBD. Edible CBD merchandise was scheduled to be available in Canada on Oct seventeen, 2019, to be used only for human consumption. Still.
European Union:
The E.C.U. The commission stated that Cannabidiol (CBD) and other cannabinoids would be categorized as “novel foods” in 2019, which means that CBD-based products would first get authorized under the European Union Novel Food Regulation states that because “CBD-based product has not been used as a main food or ingredient of food before May 15, 1997, before it is getting itself to be positioned on the market within the European Union as a main food or ingredient of the main food, a security assumption under the European Union Novel Food Regulation is required.”
The counseling applying to extracts of CBD, synthesized, and amalgamated CBD. Every one cannabidiol merchandise and oil of CBD was organized for a final statement by the commission of E.C.U. in March 2019. Still. Makers of CBD merchandise would be required to run safety tests and have to prove safe consumption, hinting that CBD merchandise wouldn’t be eligible for legitimate commerce till a minimum of 2021.
Cannabidiol is listed within the E.U. Cosmetics Ingredient info (CosIng). However, the listing of an associate ingredient, appointed with an INCI name, in CosIng doesn’t mean it’s to be employed in cosmetic merchandise or is approved for such use.
Several industrial hemp varieties are often de jure developed in Western Europe. Still. For example, an assortment, “Fedora 17,” has a cannabinoid profile reliably around 1%, with consciousness-altering drug lower than zero.3%.
New Zealand:
In 2017 the government. Created changes to the laws; thus, restrictions would be removed, which meant a doctor could visit Cannabidiol to patients. however, they contain very little or none
The passing of the Misuse of medication (Medicinal Cannabis) change Act in December 2018 means that Cannabidiol isn’t any longer a controlled drug in New island. Still. However, maybe a medicinal drug beneath the Medicines Act, with the limitation, that “the tetrahydrocannabinol (T.H.C.s) and determined substances inside the item should not surpass 2 percent of the all-out CBD, consciousness-altering drug (T.H.C.) and alternative such substances.”.
Sweden:
Cannabidiol is assessed as a medical product in Sweden.
Switzerland:
While the psychoactive drug remains embezzled, Cannabidiol isn’t subject to Swiss people Narcotic Acts as a result of this substance doesn’t manufacture a comparable mind-altering impact. Cannabis merchandise containing not up to 1% psychoactive drug is often sold and purchased de jure.
United Kingdom:
Cannabidiol, in associate oral-mucosal spray formulation combined with delta-9-tetrahydrocannabinol, maybe a product obtainable (by prescription solely till 2017) to relieve severe jerkiness. Thanks to induration (where alternative antispasmodics have not been effective).
Until 2017, merchandise containing Cannabidiol marketed for medical functions were classed as medicines by the U.K. regulatory body. The Medicines and tending restrictive merchandise Agency (MHRA) and will not be marketed while not regulatory approval for the medical claims. As of 2018, cannabis oil is legal to possess, buy, and sell within the United Kingdom, providing the merchandise that doesn’t contain over zero.3% psychoactive drug and isn’t publicized as providing a healthful profit.
In Gregorian calendar month 2019, the U.K. Food Standards Agency indicated it’d regard CBD merchandise and CBD oil as a novel food having no history of use before might 1997. Explicit that such merchandise should have authorization and well-tried safety before being marketed. The point in time for firms to register a CBD product as an associate authorized novel food with the F.S.A. is thirty-one March 2021; failure to register can exclude companies from marketing CBD.
United Nations:
Cannabidiol isn’t scheduled beneath the Convention on mind-blowing Substances or the other international organization drug treaties. Still. The World Health Organization (WHO) recommended and endorsed that CBD stayed extra in 2018.
United States:
As of March 2020, Cannabidiol extracted from marijuana remains a Schedule I Controlled Substance and isn’t approved as a prescription drug or dietary supplement or allowed for interstate commerce. CBD derived from hemp (with zero.3% psychoactive drug or lower) is legal to sell as a cosmetics ingredient. However, it cannot be sold beneath federal law as an associate ingredient in the food, dietary supplement, or animal food. It may be a common idea that the legal ability to sell hemp (which might contain CBD) makes CBD legal.
In September 2018, following its approval and recognition by the F.D.A. for rare forms of childhood encephalopathy, Epidiolex was rescheduled (by the Drug social control Administration) as a Schedule V drug to permit for its prescription use. This allows G.W. Pharmaceuticals to sell Epidiolex. However, it doesn’t apply broadly speaking, and every one alternative CBD-containing merchandise stays Schedule I medication. Epidiolex still needs rescheduling in some states before it is often prescribed in those states.
In 2013, a CNN program that included Charlotte’s Web cannabis carried expanded thoughtfulness regarding the utilization of CBD in the treatment of seizure issues. From that point forward, 16 states have passed laws to permit CBD items with a doctor’s proposal (rather than a medicine) for therapy of specific ailments.
Comprehensive Medical Cannabis
This is additionally to the 30 states that have passed comprehensive medical cannabis laws, which permit the employment of cannabis merchandise with no restrictions on psychoactive drug content. Of these thirty states, eight have legalized the use and sale of cannabis products without requirement for a physician’s recommendation. As of March 2020, CBD wasn’t an associate FDA-approved drug eligible for interstate commerce, and the F.D.A. inspired makers to follow procedures for drug approval. Difference Between cbd Oil Hemp Oil
Some makers ship cannabidiol merchandise across the nation. Associate, embezzled action that the F.D.A failure to enforce in 2018. With CBD remaining the topic of associate F.D.A. Investigational new drug evaluation. This isn’t thought-about legal as a dietary supplement or food ingredient, as of March 2020. Fedeproductsawfulness has created it troublesome traditionally to analyze Cannabidiol. Cannabidiol is brazenly sold in head shops and health food stores in some states wherever sales haven’t been expressly legalized.
State and native governments may regulate Cannabidiol. To Illustrate, the Massachusetts Department of Agricultural Resources issued a rule Gregorian calendar month 2019 orientating state CBD laws with F.D.A. regulations. That implies that though recreational marijuana is legitimate within the state, CBD cannot de jure be sold in food or as a dietary supplement beneath state law.
Health concerns:
In the Gregorian calendar month 2019, the F.D.A. Issued issues regather riding the protection of Cannabidiol and stating that CBD use can cause liver injury. Interfere with the mechanisms of prescription drugs. Manufacture disorders. Or affect alertness and mood. In March 2020, the F.D.A. updated its questions of safety regarding CBD. It is acknowledging the unknown effects of extended use. Still, it affects the developing brain. Fetus. Or infants throughout breastfeeding. Whether or not or not it interacts with dietary. Supplements or pharmaceuticals. Whether or not male fertility is affected. And its possible facet effects. Like a temporary state. In February 2020, the United Kingdom F.S.A. suggested vulnerable folks. Equivalent to pregnant ladies. They are breastfeeding mothers. And people are already taking medication for alternative medical issues not to take CBD. The F.S.A. any suggested that healthy adults shouldn’t consume over 70 mg CBD per day. Still.
2018 Farm Bill and hemp:
The 2014 Farm Bill legitimized the sale of “non-viable hemp material” grown Within the states, participating in the Hemp Pilot Program. Which defined hemp as cannabis containing less than 0.3% of T.H.C. Although, 2018 us Farm Bill led some states to interpret the bill as facultative non-public farmers to grow hemp. For the extraction and retail of CBD, federal agencies preserved administrative body over hemp-derived CBD as a Schedule. By federal law, non-public enterprises developing hemp-derived CBD are duty-bound to cultivate hemp solely for industrial functions that involve the fiber and seed, however not the flowering topnotch that contain psychoactive drug and CBD. Hemp CBD merchandise might not be sold into general commerce, however instead are allowed just for analysis. The 2018 bill needs that research. And the development of CBD for a therapeutic purpose would need. To be conducted beneath notification and news to the F.D.A.
F.D.A. warning letters:
From 2015 to Nov 2019, the F.D.A. Issued dozens of warning letters to yank makers. Of CBD merchandise for false advertising.
The F.D.A. aforementioned that the letters were issued. To enforce action against firms that. Were deceiving customers by promoting embezzled. Merchandise was meagerly proof of safety. In July 2019, the Federal and Drugs Administration (F.D.A.) stated that “Selling unapproved products with unsubstantiated therapeutic claims. Still. Love claims that CBD merchandise will treat serious diseases and conditions. That will place patients and customers in danger by leading them to place off necessary treatment. Additionally, there are many questions about the science, safety, effectiveness, and quality of unapproved merchandise containing CBD.”
In October 2019, the F.D.A. and the F.T.C. proclaimed a joint warning to an Everglade State supplements company. It was promoting CBD merchandise as unapproved medication to treat childhood autism. It included attention-deficit upset disorder, Parkinson’s disease, Alzheimer’s disease, acne, and infant teething and earaches. Still. The warning conjointly applied to hemp CBD capsules and oil marketed lawlessly, whereas not adhering to the federal definition of a dietary supplement. Additionally, Idaho, Nebraska, and American state are the only three states of Gregorian calendar month seven, 2020. It was to ban the employment of CBD in any capability.
Mislabeling and poisoning:
A 2017 analysis of Cannabidiol or CBD content in oil, tincture, or liquid vape products purchased on-line within we showed that 69% were misbranded. With 43% having higher and 26% having lower content than explicit on product labels.
As of Gregorian calendar month 2019, 1,085 people contacted the U.S.A. poison management centers concerning CBD-induced diseases. Doubling the number of cases over the 2018 rate and increasing by nine-fold of 2017. Of cases reported in 2019! over 33% received medical attention, and forty-six folks were admitted to a hospital intensive care unit. however, they contain very little or none